Covid-19 vaccination: How India failed those who suffered from adverse reactions
The challenges faced by individuals in India who suffered adverse events following the COVID-19 vaccination. It highlights several cases where people experienced severe reactions, including paralysis, blood clots, and even death, after receiving the vaccine. However, the government’s response has been inefficient, with poor reporting, investigation, and compensation mechanisms. The article also notes that the official data on adverse events is likely underreported and that the system for handling these cases is weak at the district level. The article emphasizes the need for a more robust and transparent system to address the concerns of those affected by vaccine-related adverse events.
The article discusses how India failed individuals who suffered adverse reactions to COVID-19 vaccines, citing inadequate reporting systems, underreporting of adverse events (AEFIs), and lack of government support. Families of those affected struggled to get information, as reporting was often delayed or suppressed to avoid vaccine hesitancy. Despite over 89,000 reported adverse reactions, the article highlights cases where vital information was withheld, and families had to fight for recognition and compensation for vaccine-related deaths or disabilities.
Some of the severe adverse events experienced by individuals after receiving the COVID-19 vaccine in India include:

- Thrombosis with thrombocytopenia syndrome (blood clots and low platelet count) – This led to the death of 18-year-old Rithaika Sri Omtri. Guillain-Barré syndrome (a condition where the immune system attacks the nerves) – This was experienced by 40-year-old Sudhir Waghmare, who suffered paralysis in his limbs. Cerebral bleed/hemorrhage – This was experienced by 13-year-old Mahi Manek, who lost the ability to lift her right hand or move her right foot after vaccination.

Multisystem inflammatory syndrome – This led to the death of 20-year-old Karunya Venugopal, the daughter of Dr. Venugopal Govindan.The article mentions that major adverse events documented following Covid-19 vaccinations in India include allergic reactions, thrombosis, and paralysis.

Virologist Shahid Jameel experienced an adverse event following his vaccination. He faced challenges in reporting this issue to both the government helpline and the hospital. Jameel shared his personal experience, stating that after receiving the vaccine in March 2021, he suffered from a high fever for three days. “I reached out to a government helpline,” he recounted. “The representative on the line seemed uninformed. I then contacted the hospital where I was vaccinated, and although he promised to return my call, I never heard back.” Jameel believes that his case, despite being a minor adverse event, was likely not documented in the government’s reporting system. “Underreporting is a significant issue in India,” he remarked. Nevertheless, Jameel acknowledged, “The country is conducting vaccinations on a massive scale. Vaccination efforts extend beyond health centers to community centers, schools, and colleges. It may be challenging to track adverse events in all these locations,” he observed.

Inadequate reporting of adverse events can have serious consequences, such as delaying appropriate treatment and causing financial distress for families. This was the case for 13-year-old Mahi Manek from Ropale village, who was healthy until she received her second dose of the Covid-19 vaccine, Corbevax, at a school camp on July 1 without her parents’ knowledge. She developed a high fever that day and collapsed two days later, resulting in paralysis of her right side. Mahi has since undergone two surgeries for a brain hemorrhage, costing her family Rs 7.5 lakh, and now requires a third surgery. With their finances depleted, her family has been seeking donations for her care. Mahi remains unrecorded in the government’s AEFI database.
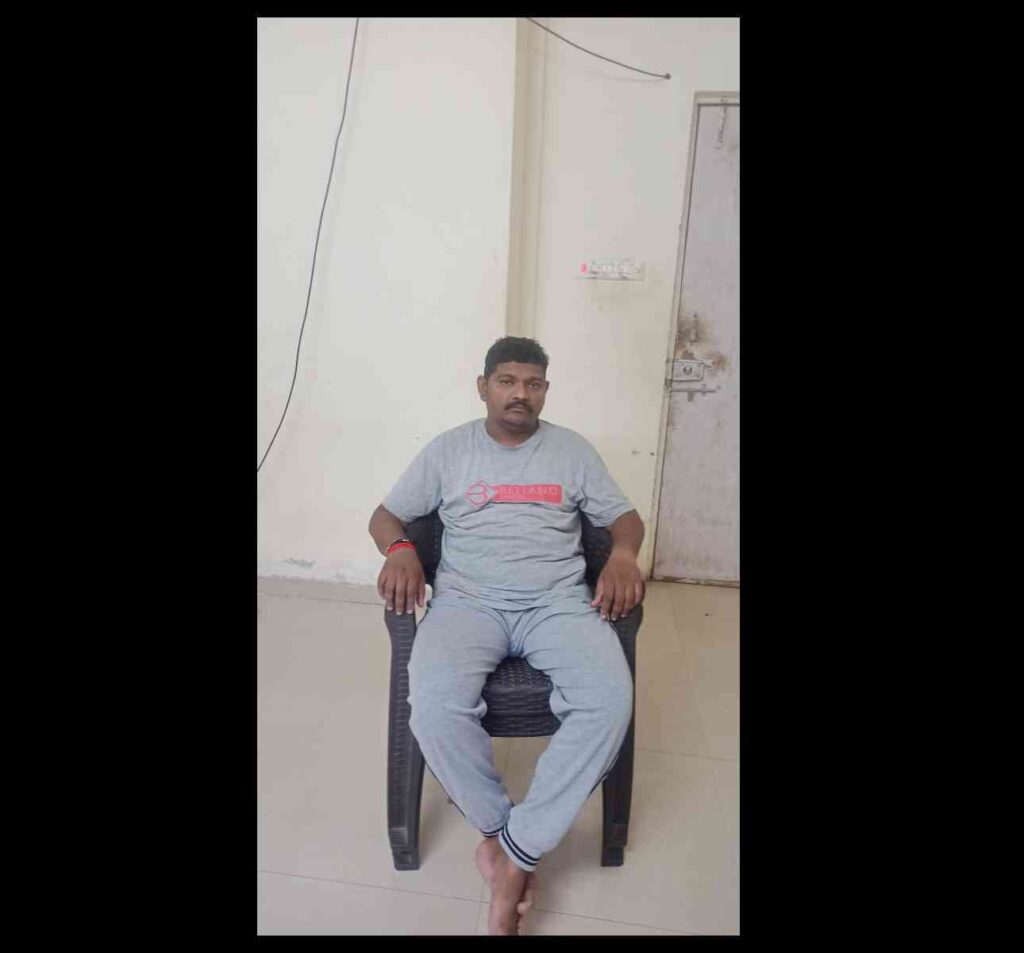
Sudhir Waghmare, a 40-year-old Pune resident, suffered severe health complications after his first Covishield vaccine dose in October 2021. Ten days post-vaccination, he experienced unusual sensations in his hands, weakness, difficulty swallowing, and balance issues, leading to paralysis within three weeks. He spent 13 days in the hospital and took six months to recover. Waghmare, who earned Rs 2,000 to Rs 3,000 daily from his tea stall, incurred medical expenses of Rs 6 lakh, depleting his savings and forcing him to take loans. Diagnosed with Guillain-Barré syndrome, a known adverse effect of the AstraZeneca vaccine, he attributes his condition to the vaccination.
The government’s response to adverse events following COVID-19 vaccination in India as inefficient and lacking in several aspects:
- Poor Reporting and Investigation: The reporting of adverse events is noted to be strikingly low, with concerns raised about the underreporting of cases. The investigation process is also criticized for being poorly conducted, with post-mortems not being carried out in many suspected AEFI deaths, leading to a lack of strong evidence.
- Lack of Compensation System: The article highlights that India does not have a system to compensate individuals affected by adverse events following immunization (AEFI). The absence of a compensation policy for recipients of COVID-19 vaccines is noted, and the government’s response to a Right to Information application confirmed the lack of such a policy.
- Inadequate Awareness and Dissemination: The article points out that there is not enough public awareness in India about the kinds of adverse events that may occur and the ways they can be reported. This lack of awareness contributes to challenges in obtaining information about adverse events and seeking compensation.
- Delayed and Inadequate Response: The article mentions that the investigation reports of AEFIs take several months to be published, indicating a delay in the process. Additionally, the response to requests for information about autopsies and inquiries into AEFIs has been described as uncooperative, with requests being rejected.
Overall, this portrays the government’s response as lacking in transparency, awareness, and support for individuals affected by adverse events following COVID-19 vaccination.
The Nagpur Court took cognizance of the said suit and issued a Show Cause Notice to the Adar Poonawalla, Cyrus Poonawalla, and Serum Institute.
In May 2021, 18-year-old Rithaika Sri Omtri received the first dose of a Covid-19 vaccination at a centre in the locality of Bagh Amberpet in Hyderabad. She was administered the Covishield vaccine, developed by the University of Oxford and the British-Swedish firm AstraZeneca, and licensed to the Indian firm Serum Institute of India.
The 18-year-old had just passed her twelfth grade and was planning to study architecture. “We heard that vaccination may become mandatory for office-goers and college students. Hence she went,” her mother, Rachana Gangu, told Scroll in October.
The nurse gave Omtri a jab, asked her to wait for 30 minutes, then let her leave. Gangu found this odd. Just a week earlier, a cousin of Omtri’s had received the same vaccine in London – there, the nurse had explained possible adverse effects that could result after the shot, and asked the cousin to sign a consent form.
In fact in May 2021, as a precaution, the United Kingdom government had advised against the use of Covishield, or AstraZeneca, for those below the age of 39 if an alternative vaccine was available.
But Gangu didn’t think much more of the matter at that point.
The rest of the day after Omtri’s vaccination went well. But within five days, she developed a prickling sensation in her fingers; then, a high fever. The family consulted a physician from the city’s Apollo Hospital, who suspected that Omtri was just having an allergic reaction, and prescribed her an anti-allergic medication. When her fever did not subside for a few days, he advised a blood examination. It revealed that Omtri’s platelets had dropped to a dangerous 40,000 per cubic millimetre, against a normal range of between 1.5 lakh and 4 lakh.
Eleven days later, she began to vomit, and could not walk. That night, an MRI scan showed that her brain had several blood clots and a haemorrhage in the right frontal region. Doctors in Apollo Hospital immediately performed a craniotomy surgery on her, but her condition steadily worsened from that point on.
On June 14, two weeks after her vaccination, doctors declared Omtri brain dead. Her desperate parents first explored all medical options to save her. Eventually, on June 19, they decided to donate her heart, lungs, liver, and kidneys for transplants. “In the hope of seeing her live through others,” Gangu said.
What Gangu didn’t know at that point, she said, was that her daughter had suffered a vaccine-induced thrombotic thrombocytopenia, a rare adverse event in which blood clots restrict the flow of blood into vital organs, and also result in a low platelet count. The parents only heard later of the possible link between the vaccine and thrombotic thrombocytopenia from an uncle of Gangu’s, who was also a doctor.
It was also only later that the family learnt, they told Scroll.in, that the hospital had informed the district immunisation committee that Omtri’s death was likely a result of an AEFI, or an adverse event following immunisation. This, in medical terms, is any health complication that results from a vaccine or drug, or the process of delivering either.
In fact, much before Omtri’s death, there was wider evidence of the link between the AstraZeneca vaccine and cases of thrombotic thrombocytopenia across the world. Beginning March 2021, several European countries, starting with Denmark, suspended its use over these concerns. By April, early estimates suggested that one in every 1,00,000 people who were administered the vaccine suffered these complications.
In India, however, there was limited awareness of the problem. The government, as well as the Serum Institute of India, had published information about AEFIs at that point, but thrombotic thrombocytopenia had found no mention as a possible outcome.
Further, data suggests that in India, the system of reporting cases and collating data on AEFI, which is crucial to devising strategies to deal with them, is faring poorly.
India launched its Covid-19 vaccination programme on January 16, 2021. It has so far administered 2.1 billion doses to more than a billion people, making it the second-largest Covid-19 vaccination drive globally, after China’s. According to information on CoWIN, the government portal that records daily vaccinations, adverse reactions have so far been noted in 0.006% of all vaccine doses administered in India. Across the world, countries have reported far higher AEFI rates. Argentina has reported AEFIs in 0.06% of vaccinations, ten times more than India. Canada, Brazil and Colombia’s AEFI rate is 0.05%, eight times higher, while Chile and Paraguay reported AEFI in 0.03% of vaccinations, five times higher than India. Dr Jacob Puliyel, a former member of National Technical Advisory Group on Immunisation, or NTAGI, which advises the Indian government on vaccines, noted that this discrepancy was unusual, since it is unlikely that “the Indian population is immune to adverse effects of vaccines”. Puliyel concluded, “There is underreporting.”
In fact, according to data that the health ministry shared with Scroll.in, the country has so far recorded 89,231 instances of AEFI in response to the Covid-19 vaccination, of which 1,148 resulted in deaths. The total number accounts for only 0.004% of the 2.1 billion doses administered up to October 29 – an even lower rate than that stated on CoWIN.
There are also significant discrepancies within India. According to health ministry data that Scroll.in procured through a Right to Information application, across India, Kerala has reported the most AEFI cases – a total of 490, including 242 deaths. The most populous state Uttar Pradesh, which has administered six time more doses of vaccine than Kerala, has reported less than half this number, with 159 AEFIs, including 85 deaths.
Dr NK Arora, who heads the national expert group on vaccination administration, agreed that the apparent lower rate of AEFI in India could be a result of underreporting. “In India, data shows most adverse events are reported within the first seven days,” he said, adding that those that occur later than are often not reported. “In Western countries, adverse events that occur up to 28 days are usually reported,” he noted.
“There is no doubt that a vaccine’s benefit outweighs its risks,” said Malini Aisola, a public health activist. “But when vaccination began, districts avoided reporting adverse events with the excuse to ostensibly avoid vaccine hesitancy in people.”
Research suggests that there is not enough public awareness in India on the kinds of adverse events that may occur, and the ways that they can be reported. A Bengaluru study found that 76.5% of 217 Covid vaccine recipients who suffered an AEFI did not report it. “It is, therefore, important to take up more awareness campaigns about reporting of AEFIs through the CoWIN,” the study said.
Given this lack of awareness and poor dissemination of information, it is unsurprising that Omtri’s parents struggled to obtain information about their daughter’s death. Ahead of donating her organs, a government doctor conducted an autopsy on her, as is mandatory, in Apollo Hospital. Her parents told Scroll.in that the autopsy report was not shared with them. When they asked for information about the possibility of an AEFI, they recounted, the hospital told them to approach district authorities, who had been given the relevant information.
Omtri’s father, Pavan Omtri, decided to get to the bottom of the matter. In October, he filed two Right to Information applications with the state and Central government to access information on the autopsy and the inquiry into the possibility of an AEFI. His request was rejected by both. Unwilling to give up, he filed an appeal and a third RTI application with the state in November.
A few months after Omtri’s death, her family received a new shock.
“In November we came across a medical journal which spoke about a successful organ donation of an AEFI case in Hyderabad,” Gangu said. “The similarities were right there. We were shocked that as parents we were not informed about what caused our daughter’s death, while the hospital published a report publicly.”
Finally, in December 2021, following an appeal under the Right to Information Act, the Union health ministry provided the family with an answer. They confirmed that Omtri had suffered “thrombosis with thrombocytopenia syndrome” and succumbed to “vaccine product related reaction” – that is, that the vaccine had led to her death. However, Apollo Hospital told Scroll.in that the district AEFI committee in Hyderabad had informed them that it was not a case of an adverse event. They also maintained that the hospital had informed the family about their suspicions of an AEFI when Omtri died.
As the family struggled through RTI applications, in October 2021, Gangu also filed a writ petition in the Supreme Court demanding that the government establish a protocol for early detection and treatment of AEFIs, that it set up an expert medical board to investigate her daughter’s death, and provide the family with a significant monetary compensation. This August, the Supreme Court issued notice to the Centre to respond to Gangu’s writ petition.
Gangu noted that there were some who sought to downplay the importance of AEFI deaths, because, they argue, these deaths are rare. But, she said, even if the problem only affected one individual in several thousands, “that one life also has a right to live”.
Health activists point out that a more efficient reporting system for adverse events in response to the COVID-19 vaccine would also have allowed the country to lead the world in establishing an early treatment protocol for dealing with such cases. “India was busy suppressing its data,” Aisola said. “In the initial six months, the World Health Organisation did not have access to our data on adverse events because India was evaluating at a snail’s pace.”
Aisola said that although the government had laid down a clear and systematic protocol on paper, the adverse event mechanism remains “weakest at the district level” because “district officials were either brushing aside cases of adverse events or not collecting adequate evidence to substantiate it”.
A detailed email to the Ministry of Health and Family welfare about the lack of data, poor reporting and investigation into AEFIs, had yielded no response as of the time of publishing.
This story is part of Common Ground, our in-depth and investigative reporting project. Sign up here to get a fresh story in your inbox every Wednesday.
A minor AEFI, according to health ministry guidelines, can include symptoms such as fever, vomiting, body pain and swelling. A severe AEFI can include high grade fever, and more swelling – but can typically be managed without hospitalisation. A serious AEFI usually involve conditions for which a person needs hospitalisation, and which may lead to disability or death. So far, major adverse events documented following Covid-19 vaccinations include an allergic reaction called anaphylaxis, thrombosis and paralysis.
The eminent virologist Dr Shahid Jameel, who was head of the Indian Sars-CoV-2 Genomics Consortium, a government panel to study mutations in the coronavirus, said that most Western countries had a well-established system to inquire into AEFI cases. “They capture the data, record it and also make this information public,” Jameel said. “In India this system does not work well.”
Jameel cited his own example. He was vaccinated in March 2021. For three days after, he had high fever. “I called a government helpline number,” he said. “The guy at the other end did not know anything. Then I called the hospital where I was vaccinated. He said he will call back, but he never did.”
Jameel suspects his case, although a minor adverse event, was never officially recorded in the government system. “Underreporting is a big problem in India,” he said.
Jameel added, however, “But the country is doing vaccination on such a large scale. Vaccination is not just in health centres but elsewhere too” – in community centres, schools, colleges. “It may not be possible to capture adverse events everywhere,” he noted.
An individual experiencing an adverse event can report it through one of a few different channels. They may, for instance, report it to the local district immunisation officer, or to the vaccination site where they received the jab. Alternatively, they can approach the Indian Pharmacopoeia Commission, or the vaccine manufacturer. Government workers and officials, meanwhile, can report cases on a portal known as Safe-Vac.
In May, the Central government also enabled reporting by the public on CoWIN, after the Supreme Court directed it to make the process easier for patients and doctors. The court also directed the Centre to publish data on AEFI cases publicly, while ensuring confidentiality – the government is yet to act on this.
Coimbatore-based Venugopalan Govindan, aged 51, who suffered hyperthyroidism and Graves’ ophthalmopathy after his vaccination last year, and registered his AEFI through CoWIN, described the portal as “a black hole”. He noted, “Nobody called me to ask for symptoms or medical reports. There is no acknowledgment if my report has been inquired. There is silence from the government.”
Aisola explained that whatever channel a person chose to report through, it was essential that the information quickly reached the district immunisation officer, who bears the responsibility of inquiring into AEFI cases. The officer has to fill a preliminary case investigation form and submit it to a state and a national committee within 10 days. These committees were created in 1988, when the AEFI surveillance system was set-up – their responsibilities include ensuring that safe immunisation procedures are followed, encouraging vaccine uptake and investigating adverse events.
The district AEFI committee has to visit the immunisation site, vaccine storage points, the patient’s residence and neighbourhood, and the treatment centre, to collect information about the patient’s health before vaccination, hospitalisation or postmortem reports, and the vaccine storage facility. The committee has to submit a final case investigation form, along with all relevant medical documents, to the state and national committee within 70 days.
The cases are classified into one of eight categories, ranging from A1, which is caused by a “vaccine product related reaction”, and A3, which is caused by an “immunization error related reaction”, to C, which refers to cases deemed “coincidental”, to D, or unclassifiable cases. Broadly, the district committee classifies minor cases and forwards severe and serious cases to the state and national committees for final classification.
But the state and national committees rely on documents and evidence collected by the district committee, and in many instances the reports submitted by the latter are incomplete, a senior World Health Organisation representative in Maharashtra said.
In fact, in Govindan’s case, the government’s lack of response to his attempt to report his AEFI was particularly distressing because he suffered a tragedy last year, and could not find closure because of a paucity of information. In July 2021, his daughter Karunya Venugopal, a data science student, died, a month after she was vaccinated. The 20-year-old suffered multisystem inflammatory syndrome and died after four weeks of hospitalization. In October, the national committee classified her death under the B1 category: cases where the AEFI has a “temporal relationship” with the vaccination, but where there is insufficient evidence to determine whether the vaccination was the cause.
In some cases, families continue to wait for the final report more than a year after losing a member to suspected AEFIs. Among them is Dr Satish Chandra, who was director at the National Institute for Mental Health and Neurosciences, or NIMHANS, in Bengaluru in 2015.
Chandra told Scroll.in that after vaccination, his brother-in-law, K Sarvottam, suffered thrombosis, and then a brain haemorrhage, and died in Bengaluru in March 2021. “I reached out to top decision makers in the health ministry when he was on a ventilator,” he said. “Till date there is no response.”
Chandra reported the case to the district AEFI committee. Sarvottam’s treating doctor, Gurucharan Adoor, even made a presentation to the committee, showing members evidence of vaccine-induced haemorrhage. “We showed them records and recommended this be categorised as AEFI,” Adoor told Scroll.in. But, Chandra said, after that they heard nothing from the government.
The health ministry’s website lists 1,527 reports of AEFIs from across India. Scroll.in analysed these and found that it typically took between three and eight months for the final reports to be published. Sarvottam’s case was not among them – suggesting that in the 18 months since his death, either his case had not been discussed in the national committee, or that it had been discussed, but that there was a delay by the AEFI secretariat in publishing its report.
Chandra said that he later learnt that vaccine-induced thrombosis can be treated with intravenous immunoglobulin. “Our intention to report this was to bring it in records,” he said. “But the local AEFI committee did not bother to visit the hospital or family to gather evidence.”
On the ground, district officers explain they are ill equipped to handle adverse event investigations when they are overseeing such massive vaccination numbers: while currently, between 3 lakh and 4 lakh people are vaccinated against Covid-19 in India each day, until last year the number was between 60 lakh and 80 lakh. “That is a huge number to follow up with,” said a health official in Nandurbar, a tribal-dominated district north of Maharashtra.
That strikingly low numbers of AEFI events are reported is apparent in the district: Nandurbar has not reported even a single serious adverse event related to Covid-19 vaccinations, despite having administered 20 lakh doses of vaccine. It reported 34 minor AEFIs up to mid-October – this accounts for a reporting rate of less than 0.002% of total vaccinations, lower even than the national average.
Puliyel said district officials fear reporting high AEFI numbers because they consider it “a big black mark on themselves”. He explained, “It could mean their vaccine storage, handling or immunisation process is at fault. No one likes to be held accountable when such a big campaign is at work.”
Until 2020, national vaccination programmes in India, such as for polio, measles and rubella, had only focused on children. The committees at the district level had paediatricians, forensic experts, and government authorities. When the Covid-19 vaccination programme was rolled out, these were expanded to include other experts, such as a gynaecologist, neurologist and cardiac specialist. But several district officials said the committee members were not given adequate training on the process.
According to Aisola, “Districts frequently discard adverse events without investigation, claiming that they were linked to comorbidities.”
The failure to adequately report these adverse events can have serious repercussions. It can, for instance, deprive patients of the right treatment at the right time and drive families deep into debt. Such was the case with 13-year-old Mahi Manek and her family in Solapur district’s Ropale village.
Manek’s family says the sixth-grade student was healthy and fit until July 1. That day, her primary school organised a Covid-19 immunisation camp in collaboration with Ropale’s primary health centre. Her parents were not informed about it. She received a second shot of Biological E’s Corbevax, which, in December 2021, was approved for emergency use in India.
Manek developed high fever the same day. Two days later, she collapsed while walking to school. Ever since, she has been unable to lift her right hand or move her right foot. Her family took her to several hospitals, in Pandharpur, Solapur, Belgaum and Mumbai. Her uncle, Chaitanya Rokade, told Scroll.in that she suffered a brain haemorrhage and was operated on twice, once in Solapur and then in Mumbai.The cost of her treatment had totaled to Rs 7.5 lakh.
Manek requires a third surgery now, but the family has run out of savings. In early September, when this reporter met her, a fragile Manek lay on a bed in Mumbai’s KEM hospital’s ward number 33. Her right hand and leg were stillparalysed. In October, she returned home, but her parents had to travel frequently to Mumbai to seek donations for her third surgery.
Manek’s case is not listed in government AEFI records. This is unsurprising, given that though Rokade informed the school principal, the latter did not report the case. “We did not know we were supposed to inform the health centre,” principal Bibhishan Patil said. “She collapsed two days after vaccine, not the same day.”
When Scroll.in contacted the health centre’s medical officer, Dr Vijay Sarade, he said that Manek had suffered a cerebral bleed. “It looks like AEFI,” he said, but added he had “not reported this as AEFI yet”. The Ropale PHC administered 77,000 doses up to early October, and had not reported any AEFIs up till then.
Manek’s mother Jyotsana believes the family will never know if the child had suffered an adverse event following immunisation. “We could either go after authorities or get her treated,” she said. “We chose the latter.”
Even when cases of AEFI are reported in India, they are often poorly investigated. “Chances of developing something severe is minimised if we have a vigilant system and provide care fast,” said virologist Dr Gagandeep Kang, from Vellore’s Christian Medical College.
Rushil Tamboli, from Awaken India Movement, which is providing people with legal assistance to seek compensation in cases of AEFI, said in many cases of suspected AEFI deaths, post-mortems are not conducted to gather strong evidence, as required byhealth ministry guidelines. “How will a committee with its members sitting in another city understand the case if autopsy is not carried?” he said. A government health official from Madhya Pradesh explained that in cases where the port-mortem is carried out, the detailed analysis of organs or tissues, known as histopathology, which is to be conducted by a government forensic laboratory, can be delayed by months, leading to a delay in evidence collection for the AEFI report.
The 1,527 reports uploaded on the health ministry’s website include minor, severe and serious cases. The last of these was uploaded five months ago – indicating either that the committee hasn’t generated any further reports, or that there is a delay in publishing them.
Arora, who heads the national expert group, said the committee meets two or three times a month. “Sometimes we discuss one case for an hour,” he said. “You may feel the progress is slow, but we do detailed evaluation.” He added that in the last one-and-a-half years, 24 “sentinel sites” have been set-up across India to improve reporting and investigations – these refer to health institutes or medical departments that have been specifically tasked with monitoring the reporting of AEFIs.
Scroll.in’s analysis of the 1,527 reports on the health ministry website revealed that in 51% cases, the adverse reaction was found to be “coincidental”, while 18% cases were found to have occurred due to an immunisation error, or vaccine product related reaction. Strikingly, the national AEFI committee found 17% of the cases to be “inconclusive” or “unclassifiable”.
Kang explained that another hurdle when it came to determining AEFIs was that India did not have baseline assessments of several specific health problems, particularly with regard to their occurrence at different ages. For instance, she said, if a 30-year-old suffers a seizure after vaccination, and the AEFI committee finds overall that the number of such seizures among that age group after vaccination is higher than in the general population of the group, it can determine that the vaccine caused the seizure. “But in absence of baseline data, we cannot say that the vaccine is 100% responsible,” she said. “Seizures could be caused by a number of factors. That is where the problem begins.”
The problems with India’s handling of Covid-19 AEFI cases are compounded by the fact that the country does not have a system to compensate those who are affected.
Scroll.in filed a Right to Information application seeking details of the government’s compensation policy for AEFIs resulting from Covid-19 vaccinations. The health ministry responded that “there is no policy for compensation for recipient of Covid-19 vaccines after its approval against any kind of side effects or medical complications that may arise due to inoculation.” An email to Serum Institute of India and Bharat Biotech, the manufacturer of Covaxin, the other of the two most common vaccines used in India, yielded no response.
In March 2021, the World Health Organisation introduced a “no-fault compensation programme” as part of its Covax initiative, which seeks to ensure equitable access to vaccines across the world. Under this programme, for vaccines procured through the initiative, Covax provides a lump-sum compensation to those suffering serious adverse events in 92 low and middle income countries. The programme protects manufacturers from liability – in recognition of the fact that they had to deliver a vaccine quickly, and could not spend years to assess clinical safety data.
Though India is listed among the 92 countries, a health ministry official told Scroll.in that it had only received a small quantity of vaccine under the initiative, and had not accessed any compensation under it.
In some countries, manufacturers sought indemnity from different governments before entering their market. Many countries, including the United States, United Kingdom, Canada, and most countries in the European Union, provided this indemnity to manufacturers, essentially taking on the responsibility of dealing with AEFI cases themselves.
India did not sign such an indemnity clause with manufacturers. But so far, a senior government official confirmed, the government has not directed any manufacturer to pay compensation to any patient who had suffered an AEFI following a Covid-19 vaccination.
Government officials told Scroll.in that the Central government had assured free treatment to those who suffered adverse events – but on the ground, patients have received little support from government hospitals.
Sudhir Waghmare, who is 40, and who lives in Pune, is a case in point. In October 2021, Waghmare received his first shot of Covishield at a Pune government school. Ten days later, he felt like there was “current passing through his hand”, then he felt weak, and found himself unable to swallow food or to balance properly while walking. Within three weeks of vaccination, he experienced paralysis in his limbs, and could no longer hold a kettle at his stall. He was hospitalised for 13 days, then required home-rest for six months to recuperate.
Waghmare owns a tea stall in a busy timber market in the city, and earlier managed to earn between Rs 2,000 and Rs 3,000 per day. But his visits to numerous doctors, and his various treatments, ate up Rs 6 lakh. He exhausted his savings, and borrowed money from friends and family.
He blames the vaccine for the sudden paralysis attack. “Nothing else explains it,” he said. “I had no other health complications before that.”
Waghmare suffered Guillain-Barré syndrome, an adverse event well documented in some recipients of the AstraZeneca vaccine. A United Kingdom study noted that among those who received a first dose of the vaccine were at greater risk of contracting this syndrome than those who received other vaccines.
Waghmare said that after he began experiencing these symptoms, he returned to the vaccination site and informed the nurse. The nurse asked him to visit a municipal hospital but did not report it on CoWIN. Even as he struggles to get make ends meet again, Waghmare believes that he should be provided “compensation for the medical bills I incurred and the months I could not work due to treatment”.
This reporting was supported by a grant from the Thakur Family Foundation. Thakur Family Foundation has not exercised any editorial control over the contents of this article.
Ref: https://scroll.in/article/1036361/how-india-failed-those-who-were-harmed-by-the-covid-19-vaccine
EXCLUSIVE: 80 of the most common adverse events reported after COVID-19 vaccination – October 13, 2021

An original analysis
The following is an exclusive analysis of illnesses and disorders occurring after Covid-19 vaccination as reported to Vaccine Adverse Event Reporting System (VAERS), a federal database. It does not include all of the adverse events reported; it summarizes some of the most common or problematic categories.
Report an adverse event after vaccination online here.
A report does not necessarily prove the illness or death was caused by the Covid vaccine. The system is designed to collect adverse events after vaccination to uncover any patterns not captured during vaccine studies.
Read CDC info on COVID-19 vaccine here.
Scientists have estimated that adverse events caused by medicine occur at a rate up to 100,000 times higher in the general population than what’s officially reported, since it’s established that most adverse events aren’t reported to the database. However, some claim Covid-19 vaccine adverse events are not as likely to be underreported, due to close monitoring and widespread publicity surrounding Covid-19 vaccination.
According to the Centers for Disease Control (CDC) and Food and Drug Administration (FDA), the benefits of Covid-19 vaccination outweigh the risks for all groups and age categories authorized to receive it.
Note: underlined items link to related studies.
Covid-19 Vaccine: Analysis of Common Illnesses Reported to Federal Vaccine Adverse Events Reporting System (VAERS)
As of Sept. 27, 2021
1. 226,457 Temperature regulation, fever (88,547), chills (78,432), sweating, flushing, hypo- or hyper-thermia
2. 174,793 Skin-related including:
154,866 Itch, rash, hives, reddening, welts, angioedema
10,691 Necrosis, swelling, infection, injury, issues
6,862 Burning sensation
2,059 Blistering, pemphigoid/pemphigus
315 Psoriasis, autoimmune skin disease
3. 164,200 Movement, muscle, nerve, neuropathy, numbness, paralysis-related including:
92,742 Movement, muscle including:
34,696 Myalgia, muscle pain
16,858 Lymphadenopathy, enlargement of lymph nodes
16,453 Muscle spasms, atrophy, injury, abnormal, swelling, bleeding, twitching, necrosis, tightness, weakness, stuff muscle rigidity with tremors or jerks (often with Parkinson’s)
10,886 Tremor, head titubation (62)
12,258 Gait disturbance, coordination abnormal, no control of limbs, unsteady steps, balance disorder, walking disability, movement disorder, dyskinesia, tics
7,962 Musculoskeletal chest pain, discomfort, injury, disorder, stiffness, fibromyalgia (230), polymyalgia rheumatica (169)
8,022 Mobility decreased, in injected limb (1,246)
673 Hypokinesia, partial or complete loss of muscle movement
407 Blepharospasm, tight closure of eyelids
248 Motor dysfunction, apraxia
206 Restless leg or arm syndrome
189 Hypotonia, reduced muscle tone or strength
186 Trismus, inability to open mouth or jaw
145 Rhabdomyolysis, skeletal muscle breakdown
116 Braedykinesia (slowed movement, as in Parkinson’s)
108 Myasthenia gravis: autoimmune disturbance of communication between muscle and nerve
61 Trigger finger, locking of finger, popping, pain
56 Areflexia: absence of deep tendon reflexes
32 Freezing phenomenon (stuck)
25 Reduced facial expression
13 Tourette’s
66,658 Nerve, neuropathy, numbness, paralysis including:
31,220 Abnormal sensation: paraesthesia, prickling, pins, needles, bug sensation (including in mouth); hypersensitivity (3,941), pharyngeal hypo/aesthesia (loss of sensation) (1,277), foreign body (940), electric shock (480)
22,478 Numbness, hypoaesthesia, oral (4,245)
7,970 Paralysis including:
5,273 Face paralysis, paresis, Bell’s palsy (2,835)
3,861 Neuralgia, neuropathy, nerve pain, injury, inflammation, nerve signal problems, symptoms, sciatica nerve injury or problems (298), trigeminal (188), post-herpatic neuralgia (40), complex regional pain syndrome (23)
1,597 Paralysis, parapalesis, hemiparesis/hemiplegia paralysis or weakness on one side (852), monoplegia in one limb (224), dyplegia among corresponding body parts (69)
696 Guillain Barre Syndrome paralysis
695 Myelitis inflammation of spinal cord or spinal cord disorder (354), weakness, paralysis; multiple sclerosis, autoimmune or relapse (199); demyelination (61); acute disseminated encephalomyelitis (ADEM), or acute demyelinating encephalomyelitis (25): autoimmune disease marked by a sudden, widespread attack of inflammation in brain and spinal cord, also attacks central nervous system and damages myelin insulation which destroys the white matter triggered by a viral infection or vaccinations; autoimmune chronic inflammatory demyelinating polyradiculoneuropathy (27); autoimmune demyelinating polyneuropathy (18); subacute inflammatory demyelinating polyneuropathy (11), autoimmune demyelinating disease (6)
500 Eye-related: gaze palsy, inability to move both eyes in same direction (249); optic blood supply nerve issues (131); cranial nerve disorder or paralysis causing pain, tingling, numbness, weakness, or paralysis of face including eyes (49); VIth nerve paralysis: impacts 6th cranial nerve responsible for muscle that turns out the eye (40); optic neuropathy (33); IIIrd nerve paralysis: impacts the 3rd cranial nerve and can impair eye movements, the response of pupils to light, or both (31)
254 Mastication disorder/pain (muscle used to chew food)
66 Peroneal nerve palsy (calf nerve paralysis with tremors)
62 Parkinson’s disease, Parkinsonism
32 Vocal cord paralysis
13 Acute neuropathy
1 Acute flaccid myelitis
Read more analysis in my article here at JustTheNews.com
4. 139,395 Pain, discomfort, tenderness, location not specified or extremity (56,411)
5. 137,499 Feeling “abnormal,” tired, fatigue, malaise, weak, chronic fatigue syndrome
6. 121,538 Injection site bleeding, bruising, warm, weakness, swelling, pain, rash, issues
7. 111,549 Headache including migraine, thunderclap
8. 80,416 Heart events (other than heart attack and bleeding)
32,804 Heart rate abnormalities, palpitations, fibrillation, flutter, arrhythmia, extrasystoles, tachycardia
30,217 Chest pain, discomfort
8,502 Abnormal blood pressure: hypertension (5,252), hypotension (3,250)
2,898 Myocarditis (inflammation of heart muscle) (1,725), Pericarditis (inflammation of membrane surrounding heart) (1,142), Endocarditis (inflammation of heart inner lining) (31)
1,697 Arrest (abrupt loss of heart function), cardiac death (39)
1,619 Myocardial swelling, rupture, necrosis, irregularity
930 Ventricular problems
833 Cardiac failure: acute, chronic, congestive, left or right
538 Severe chest pain from inadequate blood supply to heart (angina pectoris)
378 Pericardial effusion (fluid)
9. 77,976 Lung and respiratory-related
40,056 Respiratory abnormalities, cough (15,036), failure (721), pain, distress, obstructive, infection, virus, rhinitis-related, sinusitis and sinus-related including:
5,033 Rhinorrhoea, nasal thin mucus discharge, other rhinitis, pain
4,373 Nasal congestion
2,779 Nasopharyngitis (a cold)
635 Nasal Polyps, disorder, swelling, injury, blister
38,956 Lung, pulmonary other than clots or bleeding including:
31,426 Dyspnoea (shortness of breath that can be linked to asthma, heart failure and lung disease)
3,554 Pneumonia
1,220 Asthma
471 Water on lungs (pleural effusion), acute edema (17)
430 Pain
326 Inflammation
315 Chronic Obstructive Pulmonary Disease
185 Mass
10 Traumatic lung injury
10. 76,837 Dizzy, fainting, fall, concussion, vertigo, loss of consciousness
11. 60,655 Nausea
12. 48,882 Arthritis; joint or bone pain including jaw (1,718)
13. 40,415 Abdominal pain, discomfort; gastric inflammation, problems; intestine or colon injury, colostomy; diarrhea (19,339); indigestion (1,275); diverticulitis-related (246); Crohn’s autoimmune disease (106); Celiac autoimmune disease (19); C-Diff (62)
14. 34,589 Covid-19 after vaccination (17,795), Covid-19 positive (13,019), complications (1,224) Covid-19 pneumonia (1,200), suspected Covid-19 (689), asymptomatic Covid-19 (426), post-acute Covid-19 syndrome (236)
15. 32,023 Blood: clots, bleeding including in heart and brain
Definitions:
Brain stroke: caused by blockage from clot/thrombus (ischemic) or bleed (hemorrhagic, caused by aneurysm).
Aneurysm: bulging or weak spot in blood vessel wall.
Thrombosis: blood clot blockage.
Embolism: when a piece of clot largely obstructs blood flow.
Thromboembolism: reduced blood flow from blood clot embolism.
Infarction: obstruction of blood supply causing tissue death.
Myocardial infarction (MI): heart attack, usually from blood clot.
13,101 Blood clots: thrombosis, thrombus, infarction
4,831 Thrombosis location not specified (3,025) or deep vein (1,806)
2,984 Pulmonary (lung): embolism (2,449), thrombosis (454), infarction (81)
2,467 Heart attack: myocardial infarction (1,697), acute (611); thrombus (72); coronary artery thrombosis (39); cardiac ventricular (24); aortic (20); coronary artery embolism (3), intrapericardial thrombosis (1)
2,026 Brain stroke, infarction, thrombosis, embolism including: transient ischemic (502); cerebral thrombosis (370); cerebral infarction (309); ischemic (264); hemorrhagic stroke (107); embolism or embolus stroke (64); lacunar infarction (50); cerebellar stroke (49); transverse sinus thrombosis (47); thalamic infarction (37); superior sagittal sinus thrombosis (33); brain stem thrombosis or infarction (31); brain stem stroke (28); lacunar (27); basal ganglia thrombosis (25); basal ganglia stroke (24); ischemic (17); thrombotic stroke (12); basilar thrombosis (11); internal capsule infarction (7); cavernous sinus thrombosis (6); hemorrhagic infarction (4); vertebrobasilar stroke (2)
171 Embolism or embolus including: location not specified or peripheral (32), femoral (2), ileac (2), paradoxical (2), portal vein (2), air (1), fat (1), jugular (1)
92 Peripheral: thrombosis (60), embolism (32)
68 Portal vein: thrombosis (66), embolism (2)
63 Spleen: thrombosis (31), infarction (31), embolism (1)
53 Mesenteric (abdomen) thrombosis
44 Eye or retinal: thrombosis (29), eye infarction (14), retinal embolism (1)
38 Jugular vein: thrombosis (37), embolism (1)
36 Subclavian: thrombosis (32), aneurysm embolism (3), embolism (1)
30 Renal (kidney): thrombosis (26), embolism (4)
27 Pelvic thrombosis
25 Limb thrombosis
20 Ovarian thrombosis
18 Carotid artery thrombosis
18 Axillary vein (armpit) thrombosis
18 Hepatic (liver): thrombosis (16), embolisation (2)
14 Vena cava: thrombosis (13), embolism (1)
8 Brachiocephalic thrombosis
8 Injection site thrombosis
6 Superficial vein thrombosis
6 Fetal placental thrombosis
5 Vascular stent thrombosis
4 Postoperative thrombosis
3 Portosplenomesenteric thrombosis
3 Graft thrombosis
2 Visceral venous thrombosis
2 Spinal cord thrombosis
2 Infective thrombosis
2 Postpartum thrombosis
2 Prosthetic cardiac valve thrombosis
1 Penile vein thrombosis
1 Catheter site thrombosis
1 Truncus coeliacus thrombosis
1 Umbilical cord thrombosis
1 Vascular access site thrombosis
189 Aneurysm
67 Brain: intracranial aneurysm (51), ruptured cerebral (16)
52 Heart: aortic (34), cardiac (8), aortic rupture (6), coronary (4)
36 Aneurysm, location not specified
8 Ruptured
6 Splenic artery
5 Peripheral artery
2 Arteriovenous fistula
4 Carotid artery
2 Mesenteric artery
2 Subclavian artery
2 Gastro
1 Pulmonary (lung) artery
1 Vertebral artery
1 Basilar artery
10,381 Other internal bleeding, hemorrhage, hematoma including:
3,891 Contusion, bruise; pinpoint, small blood vessel
2,288 Cerebrovascular accident: Brain clots, broken blood vessels
1,811 Epistaxis (nosebleed)
1,102 Vaginal hemorrhage
359 Cerebellar hemorrhage, hematoma
276 Eye bleeding (hematoma, hemorrhage)
195 Rectal hemorrhage
165 Haematemesis (vomiting blood)
134 Abdominal bleeding hemorrhagic
124 Sub hematoma hemorrhage
20 Intraventricular
16 Brain stem, intracranial, or thalamus hemorrhage
8,352 Platelet count, blood cell and clotting issues, including transfusion and including:
5,480 Anemia, pallor (red blood cell deficiency)
855 Thrombocytopenia: low platelet blood clotting disorder
103 Coagulopathy (excessive bleeding)
77 Abnormal clotting including: overactive (disseminated intravascular coagulation) (38), hypercoagulation (could cause stroke) (30)
16. 29,661 Product issues: given to inappropriate age (7,946), storage error (6,803), inappropriate product distribution schedule (5,896), dose omission issue (2,063), poor quality product (2,029), preparation error (1,559), incorrect route (738), wrong product (687), incorrect formulation (581), inappropriate site (582), administration error (460), use issue (317)
17. 31,720 Mood, memory, depression, attention, nervousness, anxiety, confusion, agitation including:
6,406 Anxiety
4,733 Confusional state, delirium, incoherent
3,188 Nervousness, jittery, restless
1,930 Hypersomnia, excessive daytime sleepiness
1,852 Disorientation
1,672 Attention disturbance
1,613 Mental status fatigue, changes; mood swings, disorders
1,188 Panic attack, disorder
1,213 Memory impairment, transient global amnesia (57)
1,167 Depression, despair including: suicide-related (228) and suicide (20)
1,079 Hallucinations, delusions
945 Irritability, agitation
821 Abnormal dreams, nightmares
709 Crying, tearfulness
769 Fear
532 Depressed, altered state of consciousness; bradyphrenia (slowed thinking and info processing)
431 Emotional-related disorders
313 Abnormal thinking
282 Anger, aggression
265 Euphoric mood, mania, manic
129 Psychogenic disorders that slow thought and movement
124 Dissociation
154 Dementia
144 Staring
110 Paranoid-related
101 Psychotic-related, psychosis
76 Frustration tolerance decreased
75 Screaming
67 Apathy
39 Personality disorder
36 Relaxation
30 Depersonalization disorder
28 Dreamy state
24 Distractibility
14 Schizophrenia-related
13 Stupor
10 Self injury ideation
18. 24,223 Swelling, location not specified
19. 23,629 Vomiting
20. 21,951 Mouth and lip injury, swelling, ulceration, pain, spasm, difficulty or pain swallowing
21. 18,427 Sleep disorders, insomnia, paralysis, attacks, terror, sleepiness, narcolepsy
22. 14,213 Hearing disorders, tinnitus (10,090), deafness (2,244), loss (1,117)
23. 13,354 Vision disorders, blurred (5,376), impaired (2,975), blindness (1,211), light or color issues, altered, double, loss, abnormal
24. 10,893 Eye or eyelid pain, swelling, itch, infection, detachment, hemorrhage, movement disorder
25. 10,562 Throat irritation, tightness
26. 10,318 Menstrual-related: heavy (2,645), painful, irregular, short, absent, abnormal period
27. 9,065 Decreased appetite, abnormal weight loss
28. 9,038 Neck pain, injury, mass
29. 8,686 Taste loss, distortion, disorder
30. 8,322 Condition aggravated
31. 8,164 Death
32. 7,634 Face injury, swelling, pain
33. 7,521 Flu-like illness
34. 7,270 Brain-related, injury other than bleeding or stroke
4,444 Epilepsy-related, seizure, temporal lobe
1,518 Communication disorder or aphasia (loss of ability to understand speech due to brain damage)
558 Cognitive disorder
364 Encephalitis, brain injury
117 Meningitis-related, brain swelling
117 Fine motor skill dysfunction
74 Tonic clonic seizure, convulsion
37 Brain nerve pain, occipital neuralgia
26 Brain death
15 Brain tumor
35. 6,339 Herpes-related, shingles (5,414), chicken pox (68)
36. 6,055 Tongue swelling, abnormal, blister, paralysis, spasm, cyst
37. 6,036 Ear congestion, discomfort, infection, disorder
38. 4,940 Hypertension, high blood pressure
39. 4,937 Dry, thirsty, dehydrated
40. 4,918 Axillary (armpit) pain, mass
41. 4,539 Impaired work ability
42. 4,527 Immediate post-injection reaction
43. 3,557 Bladder, urinary tract infection, incontinence, discomfort
44. 3,412 Smell loss, disorders
45. 3,187 Speech disorders, loss of ability, hoarse, discomfort, stammering, stuttering
46. 3,013 Kidney injury, diabetes, abnormalities, failure
47. 2,210 Inflammation, not specified
48. 2,056 Breast pain, swelling, tenderness
49. 2,023 Oxygen abnormal, hypoxia, brain damage
50. 1,844 Pregnancy-related
1,003 Fetal issues: spontaneous abortion (752), death (78), stillbirth (30)
265 Uterine problems cyst, disorder, swelling, mass, pain, rupture
101 Ovarian disorders, cyst, rupture, necrosis, failure
96 Placental issues: premature separation of placenta, premature rupture of membranes, thrombosis, retroplacental hematoma detachment with bleed and C-section
79 Premature baby
72 Fetal heart rate abnormal
64 Suppressed lactation
54 Premature labor
45 Hemorrhage in pregnancy
35 Preelampsia high blood pressure
13 Complication of, or delivery
13 Postpartum hemorrhage
2 Popstpartum thrombosis, blood clot
2 Ruptured ectopic pregnancy
51. 1,803 Anaphylactic reaction or shock
52. 1,724 Induration, hardening of soft tissue
53. 1,408 Peripheral coldness (limbs)
54. 1,281 Sneezing
55. 1,073 Canker sores
56. 1,025 Oral herpes
57. 1,023 Tooth-related issues
58. 926 Appendicitis, perforated, appendectomy
59. 783 Sensory disturbance
60. 731 Hair loss
61. 725 Genital-related, pain, swelling, discomfort, rash, cyst, burning, enlarged, bleeding (353); vulva (372); testicular or scrotal pain, swelling, discomfort, disorder (247); penile (56)
62. 693 Vein disorders, vasculitis
63. 675 Sepsis, damaging response to infection
64. 627 Groin or pelvic pain
65. 567 Cancer-related including: lymphoma (225), leukemia (134), metastatic (49), white blood cell (20), prostate (18), colon or colorectal (20), pancreatic (12), thyroid (10), ovarian (8)
66. 552 Glossitis and glossdynia, burning mouth or tongue
67. 522 Liver failure, issues
68. 478 Thyroid pain, mass, hypo- or hyper- thyroidism
69. 468 Hyperaesthesia, increased sensitivity of one or more senses
70. 438 Lupus, misc. autoimmune-related
71. 338 Mechanical ventilation
72. 306 Pancreas problems, failure, pancreatic, not counting cancer
73. 277 Salivary gland pain and issues, cyst, hyper secretion, mass
74. 153 Spleen disorders
75. 83 Multiple organ dysfunction syndrome
76. 79 Adrenal, endocrine cyst, mass, bleeding, disorder, Addison’s disease (27)
77. 72 Coma
78. 60 Ulcer
79. 20 Multisystem inflammatory syndrome in children
80. 11 Children or males abnormally growing female breasts, gynecomastia
(WATCH) Follow the Science
Also Watch : Cover Story: 5G
Source: Scroll, Sharyl Attkisson, Fullmeasure
Also Read: