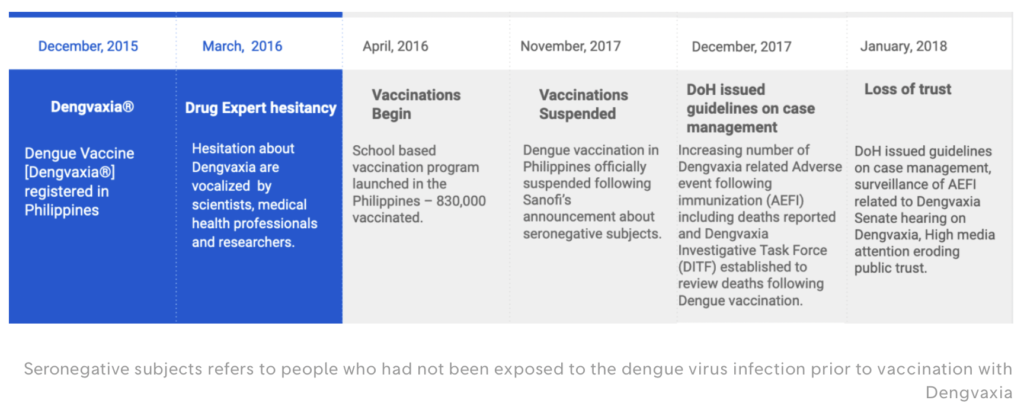
In December 2015, the president of the Philippines, Benigno Aquino III, along with others, made an agreement with the pharmaceutical company Sanofi to buy three million doses of Dengvaxia, the very first vaccine approved for dengue. The idea was to provide a million schoolchildren, who were nine years old, with three doses of the vaccine each, protecting them from the severe effects of dengue such as shock, organ failure, and death.

The Dengvaxia® vaccine by Sanofi took more than 20 years to develop and cost over 1.5 billion U.S. dollars. But making the vaccine was tricky. The antibodies from the Dengue vaccine could make the sickness worse, especially in babies and kids who haven’t had the virus before. The virus might use the vaccine antibodies to spread the sickness all over the body. So if you get dengue when you already have antibodies in your blood, it could make the sickness even worse and cause serious problems.
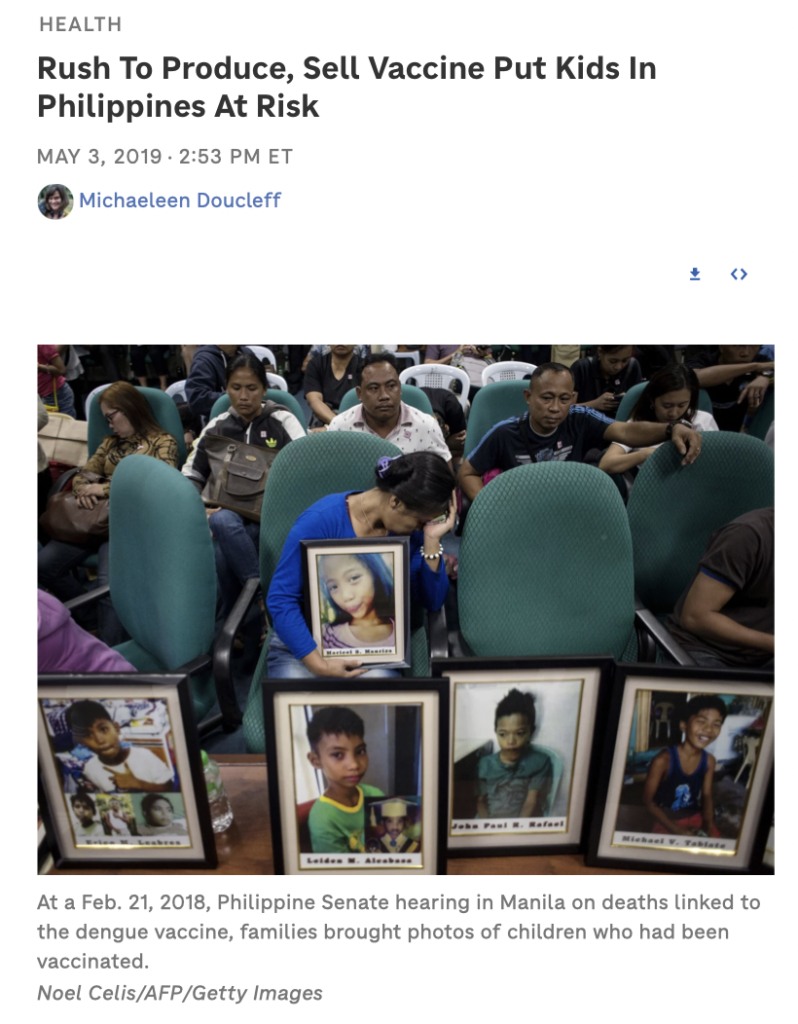
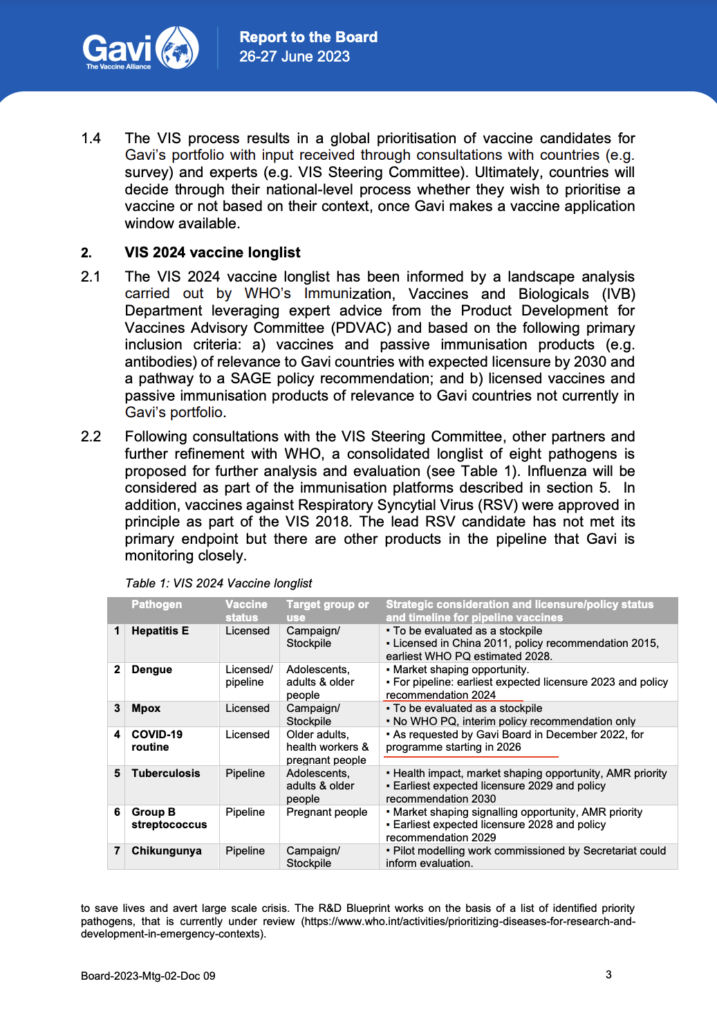
Sanofi tested Dengvaxia in many big trials with over 30,000 kids all over the world and shared the results in the New England Journal of Medicine [a]. However, there were some serious issues. Epidemiologists who looked at the data found that the trials had a lot of mistakes and missing information. Even though there was a safety signal for disease enhancement in the trials, the industry and the World Health Organization ignored it and said it was just a “theoretical possibility.” As a result, thousands of children in the Philippines got the vaccine. It was reported that around 600 children who got the vaccine have died, and the Public Attorney’s Office is investigating. The vaccine is now banned in the country.
[a] https://www.nejm.org/doi/full/10.1056/NEJMoa1506223
It’s really worrying that even though Dengvaxia caused serious harm in the Philippines, the FDA still approved it for use in the U.S. The FDA’s decision to fast-track the approval process for this vaccine raises concerns about its safety and the safety of other vaccines being developed.
Funding Ventures:
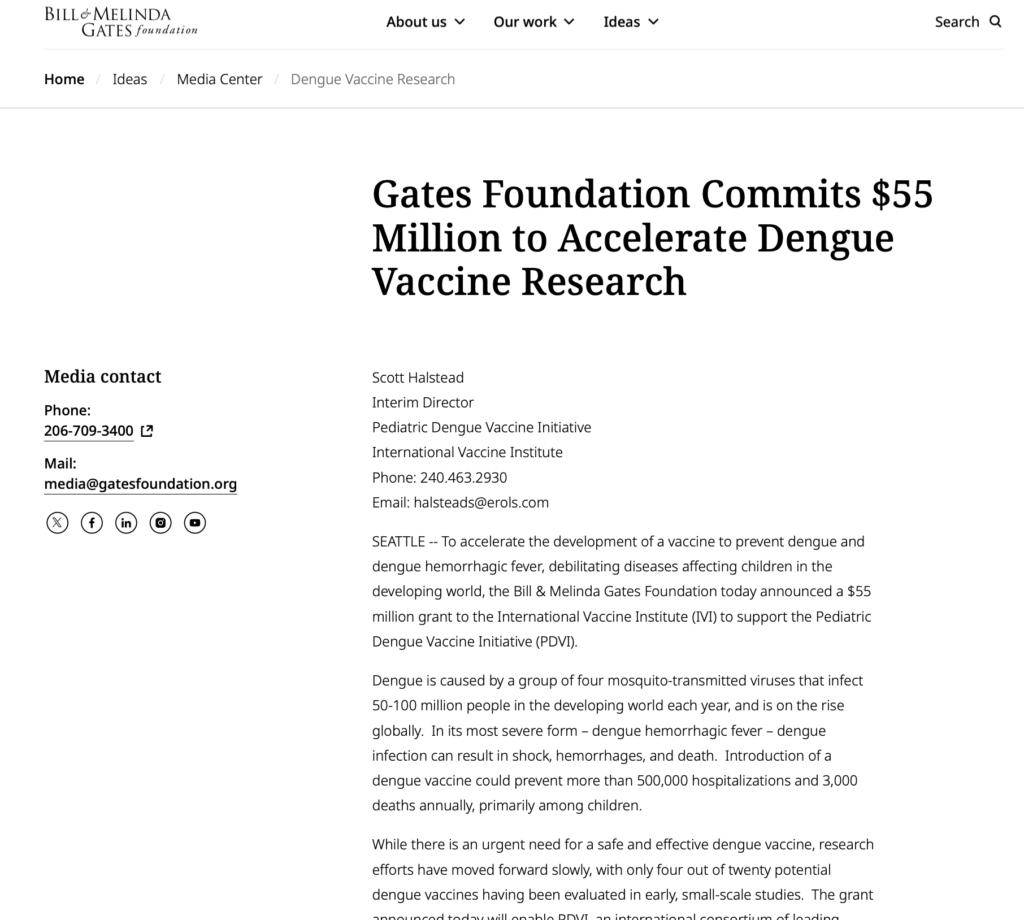
The Dengue Vaccine Initiative (DVI) has managed to attract massive sources of financing since the emergence of the Global Product Development Partnership. In 2011, the DVI received grants from vaccine developers to facilitate discussion between the major stakeholders in order to ensure the vaccine is widely available in countries where dengue is prevalent. The Bill & Melinda Gates Foundation committed to a US$ 55 million grant in 2003 to the International Vaccine Institute to accelerate the development of a safe and protective dengue vaccine . In 2011, the foundation allocated an additional US$ 6.9 million grant to further promote this agenda. Since then, it has continued to support DVI.
The Bill & Melinda Gates Foundation today announced a $55 million grant to the International Vaccine Institute (IVI) to support the Pediatric Dengue Vaccine Initiative (PDVI).
“Dengue is exacting an enormous toll on the health and economic development of millions of families in the developing world,” said Dr. Samuel Katz, Chairman of the IVI Board of Trustees, PDVI’s host organization. “It is critical to assess more fully the real impact of this disease and to begin an accelerated phase of research and development to develop a safe and effective vaccine.”
PDVI, founded in 2001 with a grant from the Rockefeller Foundation to IVI, will use the grant announced today to develop at least two clinical trial sites in Southeast Asia and South America. This research infrastructure will enable researchers to move vaccine candidates rapidly into large-scale human trials. In addition, the grant will enable PDVI to examine the extent and impact of dengue in the developing world.
Exciting progress on a new dengue vaccine: http://t.co/stgXNVbVtG via @IFLScience pic.twitter.com/6G4lemqrBU
— Bill Gates (@BillGates) December 27, 2014
Bill Gates Vaccine Investment Strategies:

CDC: 14 May 2024 — Dengvaxia is recommended to prevent dengue in children aged 9–16 years
NPJ, 15 April 2023: New dengue vaccine efficacy data a relief or cause for concern?
The World Health Organization Strategic Advisory Group of Experts on Immunization (SAGE) modified its original endorsement of Dengvaxia® recommending it only be used in dengue-immune individuals
Dr. Tony Leachon, former special adviser to the National Task Force against COVID-19.
In a series of tweets, Dr. Anthony Leachon explained he merely pointed out the “cracks” in the government’s vaccination plan, including the lack of transparency in the prices of the vaccines secured by authorities.
Leachon maintained that the public deserves to know the government’s plan and get urgent results.
“We have seen cracks at the vaccination plan, lack of transparency on the pricing and turtle-paced procurement of vaccines. We deserve to know,” he said.
“People are waiting for urgent results. The day people stop bringing you their unending COVID problems or stop criticizing a failed pandemic response or vaccination plan is the day you have stopped leading them. And both would mean a failure of leadership,” he added.
He was removed from the post in June 2021 after the President was dismayed that he cast doubt on the coronavirus data released by the Department of Health.
Leachon has openly voiced his concerns about the government’s pandemic response as well as vaccine procurement efforts through Twitter.
In May 2021, he called on the government to be transparent about its vaccination acquisition program after questioning its supposed preference for drugs that don’t have sufficient safety and efficacy data. “Transparency is key. Safety is paramount,” Leachon tweeted.
It's too easy to criticize a man when he's out of favor, and to make him shoulder the blame for everybody else's mistakes. We have seen cracks at the vaccination plan , lack of transparency on the pricing and turtle paced procurement of vaccines. We deserve to know. https://t.co/f1z7OA3q9f
— Tony Leachon MD (@DrTonyLeachon) January 24, 2021

India on Track for Dengue Vaccine by 2026
India could see a dengue vaccine [b] by 2026, according to K Anand Kumar, Managing Director of Indian Immunologicals (IIL). This announcement comes as multiple companies race to develop India’s first vaccine for the mosquito-borne disease, which claimed 485 lives last year.
[b] https://www.medindia.net/news/healthwatch/new-dengue-vaccine-the-bite-back-against-dengue-211658-1.htm
[c] https://www.medindia.net/news/mosquito-borne-diseases-are-unpredictable-threat-to-mankind-162150-1.htm
The company currently sells about 1.5 billion total vaccine doses every year, and estimates a total production capacity of as much as 4 billion doses.
“And this is also important because if there is a pandemic again in the future, we can vaccinate the whole of India in a matter of three months, three to four months,” Poonawalla said.
The company is in talks with other countries and governments to utilize those facilities in the event of future outbreaks, he said, but did not provide further details on the discussions.
Poonawalla said Serum has capacity to manufacture 100 million doses of its malaria vaccine, and could scale up further depending on demand. It has already produced 25 million doses ahead of a launch in the coming months.
The company is in talks with other countries and governments to utilize those facilities in the event of future outbreaks, he said, but did not provide further details on the discussions.
Poonawalla said Serum has capacity to manufacture 100 million doses of its malaria vaccine, and could scale up further depending on demand. It has already produced 25 million doses ahead of a launch in the coming months.
The ancient mosquito-borne disease still kills more than half a million people, mainly young children in sub-Saharan Africa, every year.
Poonawalla said Serum would focus on exporting its vaccines, such as the malaria shot, to other countries, rather than sign technology transfer deals.
Serum is also testing a single-dose vaccine for dengue, another mosquito-borne, painful and sometimes fatal disease, which it developed building on research done by the U.S. National Institutes of Health.
That vaccine is in early- to mid-stage trials in India and the company expects to complete late-stage trials in the next three years, the CEO said.
Japan’s Takeda Pharmaceutical (4502.T), opens new tab also makes a dengue shot, which is available in countries like Indonesia and Thailand, as well as Argentina and Brazil, which is currently dealing with a major outbreak and not enough vaccine.
Other companies such as Indian Immunologicals are also developing vaccines against the disease.
Mosquito versus needle
The Dengvaxia experience—involving a skewed immune response and enhanced risks—raises questions “applicable to all dengue vaccine candidates” [d] and a number of other viral vaccines. One not-often-discussed consideration pertains to the “considerable differences between a wild-type [dengue virus] delivered by a mosquito versus needle administration of a vaccine,” which have the potential to elicit different immune responses. Instead of acknowledging these vaccines’ potentially unconquerable risks, why not focus on training health care workers in the provision of the supportive care known to be “very effective when delivered by experienced practitioners”? Even in severe cases of dengue characterized by vascular permeability and fluid loss, practitioners who “accurately and rapidly” replace fluids can stabilize patients’ condition—“and rather quickly”—with the result that “the vascular permeability phenomenon abruptly disappears [f].” In addition, fruitful avenues of research could include studying the environmental and immune system factors associated with the minority of cases that involve more severe dengue outcomes.
[d] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6816420/
[e] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6816420/
[f] https://www.ncbi.nlm.nih.gov/pubmed/30783665
With vaccine damage occurring in association with many different vaccines, it is unclear why so many individuals and organizations jumped on the anti-Dengvaxia bandwagon last year, but—with a rushed COVID-19 vaccine in the works—their words of warning are worth heeding. As NPR noted [g], “the debacle in the Philippines offers a key lesson for governments and manufacturers when it comes to approving and selling new vaccines: Slow down.” The dengue expert who presciently warned about Dengvaxia’s dangers put it this way [h]:
[g] https://www.npr.org/sections/goatsandsoda/2019/05/03/719037789/botched-vaccine-launch-has-deadly-repercussions
[h] https://www.ncbi.nlm.nih.gov/pubmed/28716893
Dengvaxia-enhanced disease has created a major ethical dilemma for the vaccine community, an enduring public health management crisis, and legal nightmare. Vaccines should not harm recipients, directly or indirectly. WHO and the manufacturer owe the customer a safe product.
STOP THE SHOTS!!
Sai Prasad, as Executive Director of Bharat Biotech, he oversees manufacturing, business development, and international collaborations. He has extensive experience in clinical development, media relations, and various business operations. He has been involved in the development of several global vaccines and biologics, including ROTAVAC®, TYPBAR TCV®, JENVAC®, COVAXIN®, iNCOVACC®.
During his tenure as President of Quality Operations, Mr Prasad oversaw all aspects of quality management ensuring a safe and effective product for a global audience.
While serving as Vice President at Bharat Biotech, he oversaw a number of departments, including Business Development, Project Management, Information Systems, and Global Strategic Initiatives. As a product development and commercialisation manager, he has extensive experience in vaccines and biologics. With unique skills, knowledge and expertise, Mr. Sai Prasad bridges science to business and global cultures.
Source: CIDRAP, First Draft, Gavi, Childrenshealthdefense, Reuters, Esquire -Image